A.J.Soprano
Banned
[ame]https://www.youtube.com/watch?v=EjrmOGEGQs4[/ame]
A more detailed analysis
The University of Chicago Magazine
A more detailed analysis
III. A hiccup in our tadpole past
This annoyance has its roots in the history we share with fish and tadpoles.
If there is any consolation for getting hiccups, it is that our misery is shared with many other mammals. Cats can be stimulated to hiccup by sending an electrical impulse to a small patch of tissue in their brain stem. This area of the brain stem is thought to be the center that controls the complicated reflex that we call a hiccup. The hiccup reflex is a stereotyped twitch involving a number of muscles in our body wall, diaphragm, neck, and throat. A spasm in one or two of the major nerves that control breathing causes these muscles to contract. This results in a very sharp inspiration of air. Then, about 35 milliseconds later, a flap of tissue in the back of our throat (the glottis) closes the top of our airway. The fast inhalation followed by a brief closure of the tube produces the hic.
But we rarely experience only a single hic. Stop the hiccups in the first five to ten hics, and you have a decent chance of ending the bout altogether. Miss that window, and the bout of hiccups can persist for an average of about 60 hics. Inhaling carbon dioxide (by breathing into the classic paper bag) and stretching the body wall (taking a big inhalation and holding it) can end hiccups early in some of us. But not all. Some cases of pathological hiccups can be extremely prolonged. The longest uninterrupted hiccups in a person lasted from 1922 to 1990.
Our tendency to develop hiccups is another influence of our past. There are two issues to think about. The first is what causes the spasm of nerves that initiates the hiccup. The second is what controls that distinctive hic, the abrupt inhalationglottis closure. The nerve spasm is a product of our fish history, while the hic is an outcome of the history we share with animals such as tadpoles.
First, fish. Our brain can control our breathing without any conscious effort on our part. Most of the work takes place in the brain stem, at the boundary between the brain and the spinal cord. The brain stem sends nerve impulses to our main breathing muscles. Breathing happens in a pattern. Muscles of the chest, diaphragm, and throat contract in a well-defined order. Consequently, this part of the brain stem is known as a central pattern generator. This region can produce rhythmic patterns of nerve and, consequently, muscle activation. A number of such generators in our brain and spinal cord control other rhythmic behaviors, such as swallowing and walking.
The problem is that the brain stem originally controlled breathing in fish; it has been jerry-rigged to work in mammals. Sharks and bony fish all have a portion of the brain stem that regulates the rhythmic firing of muscles in the throat and around the gills. The nerves that control these areas all originate in a well-defined portion of the brain stem. We can even see this nerve arrangement in some of the most primitive fish in the fossil record. Ancient ostracoderms, from rocks over 400 million years old, preserve casts of the brain and cranial nerves. Just as in living fish, the nerves that control breathing extend from the brain stem.
This works well in fish, but it is a lousy arrangement for mammals. In fish the nerves that control breathing do not have to travel very far from the brain stem. The gills and throat generally surround this area of the brain. Mammals have a different problem. Our breathing is controlled by muscles in the wall of our chest and by the diaphragm, the sheet of muscle that separates chest from abdomen. Contraction of the diaphragm controls inspiration. The nerves that control the diaphragm exit our brain just as they do in fish, and they leave from the brain stem, near our neck. These nerves, the vagus and the phrenic nerve, extend from the base of the skull and travel through the chest cavity to reach the diaphragm and the portions of the chest that control breathing. This convoluted path creates problems; a rational design would have the nerves traveling not from the neck but from somewhere nearer the diaphragm. Unfortunately, anything that interferes with one of these nerves can block their function or cause a spasm.
If the odd course of our nerves is a product of our fishy past, the hiccup itself is likely the product of our history as amphibians. Hiccups are unique among our breathing behaviors in that an abrupt intake of air is followed by a closure of the glottis. Hiccups seem to be controlled by a central pattern generator in the brain stem: stimulate this region with an electrical impulse, and we stimulate hiccups. It makes sense that hiccups are controlled by a central pattern generator, since, as in other rhythmic behaviors, a typical sequence of events happens during a hic.
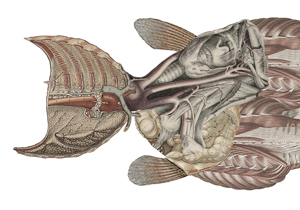
It turns out that the pattern generator responsible for hiccups is virtually identical to one in amphibians. And not in just any amphibiansin tadpoles, which use both lungs and gills to breathe. Tadpoles use this pattern generator when they breathe with gills. In that circumstance, they want to pump water into their mouth and throat and across the gills, but they do not want the water to enter their lungs. To prevent it from doing so, they close the glottis, the flap that closes off the breathing tube. And to close the glottis, tadpoles have a central pattern generator in their brain stem so that an inspiration is followed immediately by a closing glottis. They can breathe with their gills thanks to an extended form of hiccup.
The parallels between our hiccups and gill breathing in tadpoles are so extensive that many have proposed that the two phenomena are one and the same. Gill breathing in tadpoles can be blocked by carbon dioxide, just like our hiccups. We can also block gill breathing by stretching the wall of the chest, just as we can stop hiccups by inhaling deeply and holding our breath. Perhaps we could even block gill breathing in tadpoles by having them drink a glass of water upside down.
The University of Chicago Magazine










