Northwestern University scientists have developed a new bioactive material that successfully regenerated high-quality cartilage in the knee joints of a large-animal model.

news.northwestern.edu
New biomaterial regrows damaged cartilage in joints
A crucial component in joints, cartilage is notoriously difficult to repair
August 5, 2024 | By
Amanda Morris
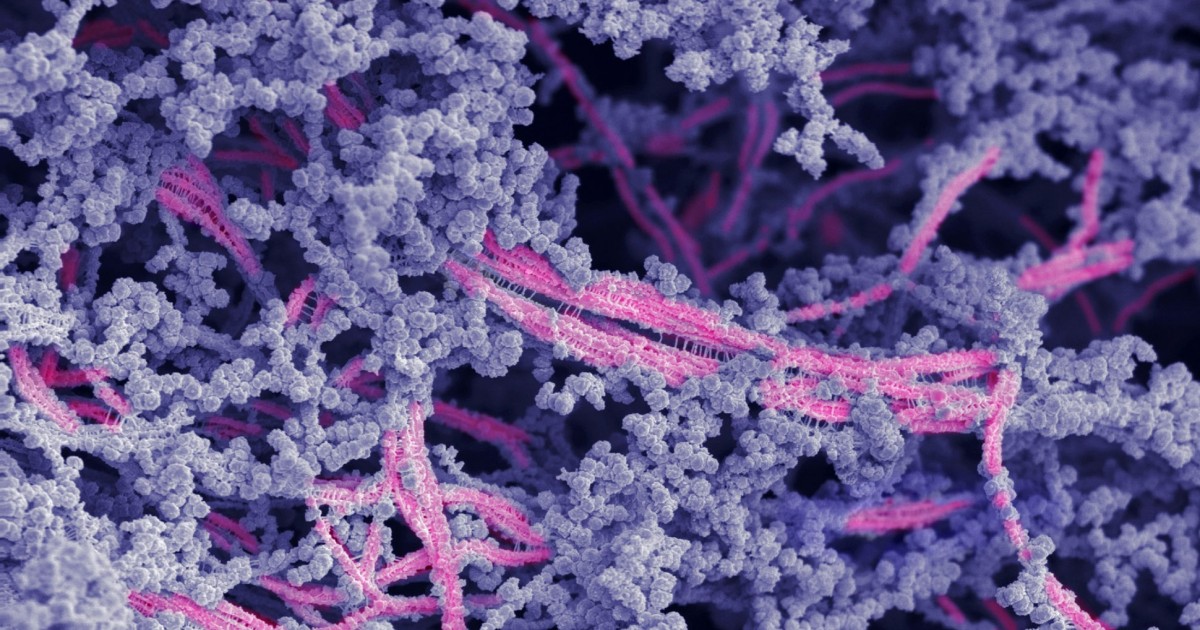
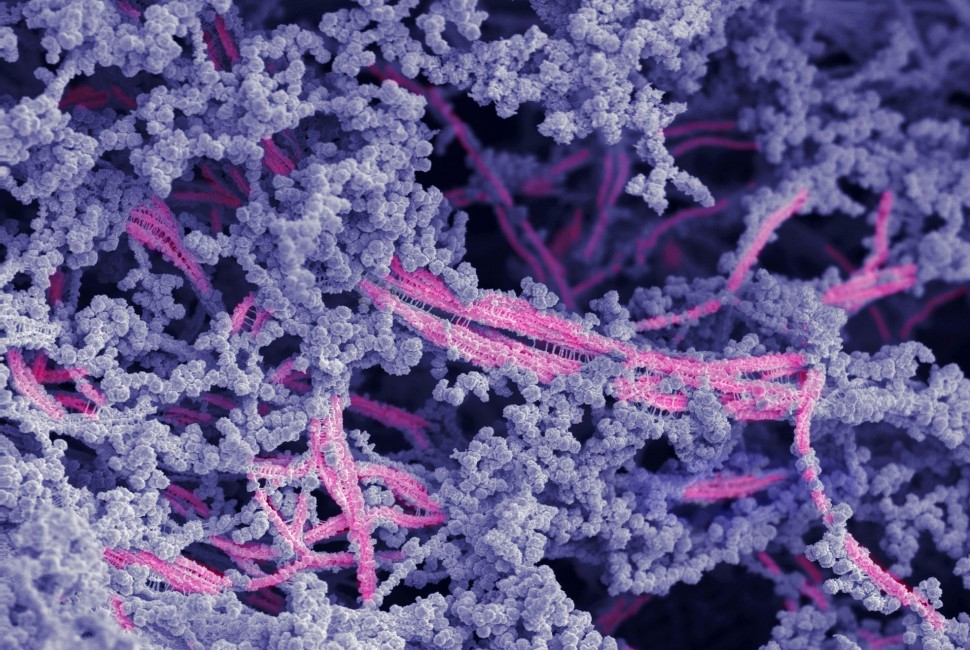
Microstructure of the new bioactive material. The fibers are in pink; hyaluronic acid is shown in purple. Image by the Stupp Group
Northwestern University scientists have developed a new bioactive material that successfully regenerated high-quality cartilage in the knee joints of a large-animal model.
Although it looks like a rubbery goo, the material is actually a complex network of molecular components, which work together to mimic cartilage’s natural environment in the body.
In the new study, the researchers applied the material to damaged cartilage in the animals’ knee joints. Within just six months, the researchers observed evidence of enhanced repair, including the growth of new cartilage containing the natural biopolymers (collagen II and proteoglycans), which enable pain-free mechanical resilience in joints.
With more work, the researchers say the new material someday could potentially be used to prevent full knee replacement surgeries, treat degenerative diseases like osteoarthritis and repair sports-related injuries like ACL tears.
The study will be published during the week of August 5 in the Proceedings of the National Academy of Sciences.
“Cartilage is a critical component in our joints,” said Northwestern’s
Samuel I. Stupp, who led the study. “When cartilage becomes damaged or breaks down over time, it can have a great impact on people’s overall health and mobility. The problem is that, in adult humans, cartilage does not have an inherent ability to heal. Our new therapy can induce repair in a tissue that does not naturally regenerate. We think our treatment could help address a serious, unmet clinical need.”
A pioneer of regenerative nanomedicine, Stupp is Board of Trustees Professor of Materials Science and Engineering, Chemistry, Medicine and Biomedical Engineering at Northwestern, where he is founding director of the
Simpson Querrey Institute for BioNanotechnology and its affiliated center, the
Center for Regenerative Nanomedicine. Stupp has appointments in the
McCormick School of Engineering,
Weinberg College of Arts and Sciences and
Feinberg School of Medicine. Jacob Lewis, a former Ph.D. student in Stupp’s laboratory, is the paper’s first author.
What’s in the material?
The new study
follows recently published work from the Stupp laboratory, in which the team used “dancing molecules” to activate human cartilage cells to boost the production of proteins that build the tissue matrix. Instead of using dancing molecules, the new study evaluates a hybrid biomaterial also developed in Stupp’s lab. The new biomaterial comprises two components: a bioactive peptide that binds to transforming growth factor beta-1 (TGFb-1) — an essential protein for cartilage growth and maintenance — and modified hyaluronic acid, a natural polysaccharide present in cartilage and the lubricating synovial fluid in joints.
“Many people are familiar with hyaluronic acid because it’s a popular ingredient in skincare products,” Stupp said. “It’s also naturally found in many tissues throughout the human body, including the joints and brain. We chose it because it resembles the natural polymers found in cartilage.”
Stupp’s team integrated the bioactive peptide and chemically modified hyaluronic acid particles to drive the self-organization of nanoscale fibers into bundles that mimic the natural architecture of cartilage. The goal was to create an attractive scaffold for the body’s own cells to regenerate cartilage tissue. Using bioactive signals in the nanoscale fibers, the material encourages cartilage repair by the cells, which populate the scaffold.
Clinically relevant to humans
To evaluate the material’s effectiveness in promoting cartilage growth, the researchers tested it in sheep with cartilage defects in the stifle joint, a complex joint in the hind limbs similar to the human knee. This work was carried out in the laboratory of Mark Markel in the School of Veterinary Medicine at the University of Wisconsin–Madison.
According to Stupp, testing in a sheep model was vital. Much like humans, sheep cartilage is stubborn and incredibly difficult to regenerate. Sheep stifles and human knees also have similarities in weight bearing, size and mechanical loads.
“A study on a sheep model is more predictive of how the treatment will work in humans,” Stupp said. “In other smaller animals, cartilage regeneration occurs much more readily.”
In the study, researchers injected the thick, paste-like material into cartilage defects, where it transformed into a rubbery matrix. Not only did new cartilage grow to fill the defect as the scaffold degraded, but the repaired tissue was consistently higher quality compared to the control.
A lasting solution
In the future, Stupp imagines the new material could be applied to joints during open-joint or arthroscopic surgeries. The current standard of care is microfracture surgery, during which surgeons create tiny fractures in the underlying bone to induce new cartilage growth.
“The main issue with the microfracture approach is that it often results in the formation of fibrocartilage — the same cartilage in our ears — as opposed to hyaline cartilage, which is the one we need to have functional joints,” Stupp said. “By regenerating hyaline cartilage, our approach should be more resistant to wear and tear, fixing the problem of poor mobility and joint pain for the long term while also avoiding the need for joint reconstruction with large pieces of hardware.”
Notes
The study, “A bioactive supramolecular and covalent polymer scaffold for cartilage repair in a sheep model,” was supported by the Mike and Mary Sue Shannon Family Fund for Bio-Inspired and Bioactive Materials Systems for Musculoskeletal Regeneration.


 I wonder who or what is behind the material.
I wonder who or what is behind the material.