--FDA unveils report showing genetically modified salmon isn't a threat to environment
--Salmon is engineered to grow faster than traditional fish
--Timeline unclear for final approval of engineered salmon
WASHINGTON--The Food and Drug Administration on Friday took a step toward approving genetically modified salmon for human consumption by releasing a draft assessment showing such production of the fish isn't a threat to the environment.
The FDA will decide whether to issue a final assessment and possibly a final approval after reviewing public comment it collects over the next 60 days, an FDA spokeswoman said. She wouldn't give an estimate on how long the process would take.
If approved, AquAdvantage Atlantic salmon, developed by AquaBounty Technologies Inc. (ABTX.LN), would be the first genetically engineered animal approved for people to eat.
The assessment concludes there is an extremely low possibility the salmon, engineered to grow twice as quickly as traditional farmed salmon, could escape from breeding and growing facilities and mate with wild or non-genetically modified fish.
The company has said nearly all of the AquAdvantage Atlantic salmon produced are sterile, but critics point out it isn't 100% and if a genetically modified fish were to mate in the wild it could pass on its altered characteristics to traditional fish.
FDA studies have shown that "only 95% of the salmon may be sterile, and the rest fertile," said Michael Hansen, a scientist with the Consumers Union, a nonprofit consumer group that also publishes Consumer Reports.
Only two AquaBounty facilities are being considered in the current FDA approval process, an egg production operation in Canada and a fish farm in Panama.
A separate determination will be made on the safety of eating the engineered salmon sometime before final approval, the spokeswoman said.
An FDA advisory panel announced its support for approval about two years ago and FDA staff, in preparation for the 2010 panel meeting, concluded the genetically altered salmon was as safe to eat as traditional Atlantic salmon, and posed little risk to the environment.
But there is still strong opposition by consumer groups who say there is insufficient evidence the engineered salmon is safe to eat.
The genetically modified salmon produce more than normal amounts of growth hormones, according to Patty Lovera, an assistant director for the Food & Water Watch group, and long-term studies need to be performed to show the effects on human health.
The company cited studies that showed abnormal hormone levels in the salmon in a submission to the FDA.
The potential for the genetically modified salmon to cause allergic reactions in humans is also a concern, Dr. Hansen said..
AquaBounty officials weren't available for immediate comment.
At a minimum, the AquaBounty salmon should be labeled as genetically modified when it is marketed, said Wenonah Hauter, the Food & Water Watch's executive director.
The FDA has said previously that there is no reason to have different labels, and that the agency may lack authority to mandate them if the product is approved as safe for people to eat.
2nd UPDATE: FDA Takes Step Toward Approving Genetically Modified Salmon
 Demonic fish. What effects does this have on the body. Are you really what u eat, friends.
Demonic fish. What effects does this have on the body. Are you really what u eat, friends. 

 I did too...although that was a nook plant in the Simpsons......
I did too...although that was a nook plant in the Simpsons......
 moment........
moment........


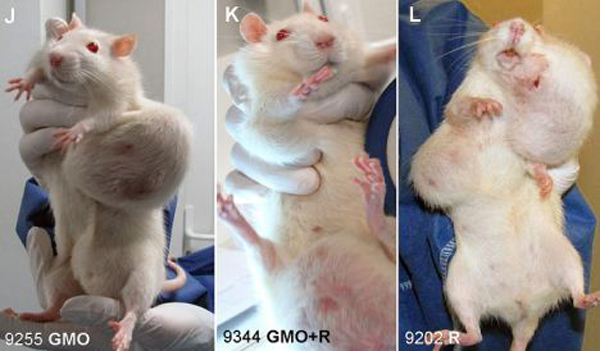
 . I just found this picture of what genetically modified/engineered food does to mammals and its disgusting...
. I just found this picture of what genetically modified/engineered food does to mammals and its disgusting...
 ... :shake:
... :shake: